Acidosis and Laminitis: Identifying Horses at Risk: EWEN Report Part 3
In this Part 3 of our exclusive report from the European Workshop on Equine Nutrition (EWEN), Dr Mariette van den Berg discusses a recent study by French researchers that evaluated if diet composition (specifically high-fibre or high-starch diets) can modulate the bacterial lipopolysaccharides (LPS) in equine faeces.
This study provided valuable information to an area of research that aims to development a reliable and simple faecal test to identify those horses who are at risk of developing acidosis or laminitis.

Feeding horses rapidly fermentable carbohydrates and abrupt dietary transitions are very common practices in the horse industry.
Gram-negative bacteria are typically your cellulolytic bacteria – fibre fermenting bacteria, or the ones that we want in the hindgut, i.e. ‘good microbes’.
Gram-positive bacteria are the lactic acid producing ones, which we label as the ‘bad’ microbes in the hindgut.
High sugar diets cause Gram-positive bacteria to overgrow and the Gram-negative die off, resulting in massive shedding of their membranes, which contain endotoxins. These endotoxins then can pass from the gut to the blood, and trigger the dangerous laminitis process.
The horse’s hindgut microbiota (the microorganisms that live in the colon and caecum) can react highly and rapidly to abrupt diet changes, with researchers observing these changes as early as five hours after feeding concentrate to horses that were previously on a hay-based diet(1).
Abrupt changes, such as when there is a rapid increase in grass sugars (water soluble carbohydrates, or WSC), or when feeding excess levels of starch-containing grain or cereal based concentrates are known risk factors for the development of digestive and metabolic disorders, such as hindgut acidosis and laminitis(2,3,4).
You may have heard of equine hindgut acidosis (acidosis means the gut content – the fluid – is acidic or has a low pH). This occurs when ‘resistant’ starch escapes digestion in the small intestine, or when the dietary starch, or WSC, concentrations are too high to be absorbed and digested before they reach the caecum.
When the undigested starch or WSC arrives in the hindgut, anaerobic bacteria ferment it rapidly and produce lactate, which quickly lowers the pH of the hindgut, resulting in hindgut (sometimes called lactic) acidosis(5,6).
Various studies monitoring changes in caecal microbial populations of the equine hindgut in response to excess starch or fructan (oligo-fructose) suggest Gram-positive bacteria, such as Lactobacillus spp. and Streptococcus spp.(5,7,8,9) play an integral role during the onset of hindgut acidosis and laminitis.
The direct pathways that trigger laminitis through this acidosis phase are still largely unclear and equine researchers around the world have suggested a variety of theories.
It seems that different active compounds (e.g. amines and endotoxins) that are released by either Gram-positive and/or Gram-negative bacteria could play a direct role (passing from the gut to blood) or an indirect role (by triggering an inflammatory response) in the lead up to a laminitic episode.
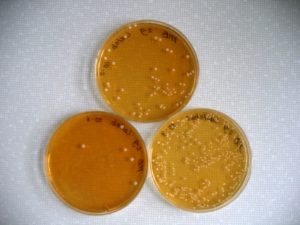 As an example, the overgrowth of Gram-postive bacteria (especially lactobacillus and streptococcus spp.) associated with lactic acid accumulation causes the death of Gram-negative bacteria (your fibre fermenting bacteria), and leads to endotoxin release. Although endotoxin causes widespread inflammatory responses in the horse, on its own it does not induce laminitis.
As an example, the overgrowth of Gram-postive bacteria (especially lactobacillus and streptococcus spp.) associated with lactic acid accumulation causes the death of Gram-negative bacteria (your fibre fermenting bacteria), and leads to endotoxin release. Although endotoxin causes widespread inflammatory responses in the horse, on its own it does not induce laminitis.
A more recent study, however, also observed an increase in specific pathogenic Gram-negative bacteria(9). The authors suggest Gram-negative bacteria develop and contribute to enterocolitis (inflammation of both the small intestine and the colon), pyrexia (fever) and lameness in the carbohydrate overload model of acute laminitis (this was a pilot study so more research is needed to replicate these results).
We do know that lactic acidosis and laminitis can severely impact the wellbeing and performance potential of horses, so monitoring the health of the gut could assist with the early detection of unfavourable changes, as well as helping identify horses at risk.
All the studies mentioned above specifically monitored the caecum of the horse, where most of the fermentation occurs (similar to the rumen of cows and sheep where fermentation takes place).
However, in practice and in a field situation, it is not possible to take samples from the caecum unless we fistulate horses (a fistula is a tubed opening through which we can collect the fluids that seep out of the digestive tract). This is a very invasive procedure that is no longer accepted in many countries and research institutions.
Therefore, researchers are turning to faecal samples as an indicator for what may be happening to microbiota in the horse’s hindgut.
This was the case for my own Master project(10), where we examined faecal pH and microbial populations in Thoroughbred horses during their transition from pasture to concentrates.
EWEN 2016 presentation
At the EWEN 2016 conference, a French research group from Agrosup Dijon (P. Grimm, J.P. Pais de Barros and V. Julliand) presented some interesting results of a study that specifically investigated particles of Gram-negative bacteria in faecal content.
As I mentioned earlier, recent results show besides Gram-positive bacteria, specific harmful (pathogenic) Gram-negative bacteria seem to proliferate in response to a high-starch diet.
The endotoxins (lipopolysaccharides, or LPS) which are found on the outer membrane of Gram-negative bacteria are composed of three structural domains (O polysaccharide, core oligosaccharide and lipid A) which can be measured with the analytical technique called mass spectrometry. (Specifically 3-hydroxymyristic acid, or 3HM, is typically used to calculate LPS concentrations.)
The resulting LPS calculations can be used to identify microbiota changes. The aim of the study by Grimm et al. was to determine if diet composition (high-fibre or high-starch) influences and changes the LPS concentrations in equine faeces.
Methodology
Six adult geldings were used in a 10-week study period that was divided into three phases.During the first phase (3 weeks), horses were fed 100% hay at 2.2% bodyweight (BW) per day. In the second phase (4 weeks), horses received a diet composed of 57% hay and 43% rolled barley in two meals. The grain component was added gradually over five days. Then, in the last 6 weeks, the horses returned to 100% hay diet without transition. The researchers collected faecal samples for analysis four hours after the morning meal on days 10 and 20 in Phase 1 and 2, and on days 10, 20 and 40 in Phase 3.
Results
The results showed LPS concentrations increased progressively when horses changed from a hay diet to hay/barley and were significantly higher 20 days after the transition.
Faecal LPS decreased significantly when horses returned to their hay diet, which was comparable to the first hay period. Nevertheless, the variability in LPS concentration between horses was larger during the hay/barley diet phase, as compared to during the hay periods.
The authors concluded LPS concentrations are very sensitive to diet changes and these alterations may reflect hindgut bacteria composition occurring under the dietary change.
The larger difference in LPS concentrations between individual animals on the high-starch diet shows some horses are more susceptible to starch than others. This is also what we observed in our study10 and highlights the need to include analysis within animals.
Additionally they suggest in future researh, LPS concentrations should be analysed in conjunction with the changes in gram-negative and gram-positive bacteria. The authors also noted only 3HM was used for the analysis and suggest more lipids fractions should be incorporated in the analysis for greater profile of gram-negative bacteria in the faeces of horses.
Nonetheless, they suggest 3HM (for LPS concentration) could be a simple method to assess the dynamics of bacterial profile in the faeces of horses; providing insight into bacterial balance or imbalance.
This is a fascinating area of research that could lead to the development of a reliable faecal test to identify animals that are at risk of acidosis and/or acute laminitis episodes.
For more information check the EWEN 2016 website (http://ewen2016.com/). Proceedings are released under ISSN: 1629-0763.
References:
1.
de Fombelle, A., Julliand, V., Drogoul, C., Jacotot, E., 2001. Feeding and microbial disorders in horses: 1 – Effects of an abrupt incorporation of two levels of barley in a hay diet on microbial profile and activities. Journal of Equine Veterinary Science 21, 439-445.
2.
Garner, H.E., Moore, J.N., Johnson, J.H., Clark, L., Amend, J.F., Tritschler, L.G., Coffmann, J.R., Sprouse, R.F., Hutcheson, D.P., Salem, C.A., 1978. Changes in caecal flora associated with onset of laminitis Equine Vet J 10, 249-252.
3.
Goodson, J., Tyznik, W.J., Cline, J.H., Dehority, B.A., 1988. Effects of an abrupt diet change from hay to concentrate on microbial numbers and physical environment in the caecum of the pony. Appl Environ Microbiol 54, 1946-1950.
4.
Longland, A.C., Byrd, B.M., 2006. Pasture nonstructural carbohydrates and equine laminitis. J Nutr 136 (Supplement) 2099S-2102S.
5.
Garner, H.E., Hutcheson, D.P., Coffman, J.R., Hahn, A.W., Salem, C., 1977. Lactic acidosis: a factor associated with equine laminitis. . J Anim Sci 45, 1037-1041
6.
van Eps, A.W., Pollitt, C.C., 2006. Equine laminitis induced with oligofructose. Equine Vet J 38, 203-208.
7.
Bailey, S.R., Baillon, M.L., Rycroft, A.N., Harris, P.A., Elliott, J., 2003. Identification of equine cecal bacteria producing amines in an in vitro model of carbohydrate overload. Appl Environ Microbiol 69, 2087-2093.
8.
Milinovich, G.J., Trott, D.J., Burrell, P.C., Croser, E.L., Al Jassim, R.A.M., Morton, J.M., van Eps, A.W., Pollitt, C.C., 2007. Fluorescence in situ hybridization analysis of hindgut bacteria associated with the development of equine laminitis. Environmental Microbiology 9, 2090-2100.
9.
Moreau, M.M., Eades, S.C., Reinemeyer, C.R., Fugaro, M.N., Onishi, J.C., 2014. Illumina sequencing of the V4 hypervariable region 16S rRNA gene reveals extensive changes in bacterial communities in the cecum following carbohydrate oral infusion and development of early-stage acute laminitis in the horse. Vet. Microbiol 168, 436-441.
10.
van den Berg, M., Hoskin, S.O., Rogers, C.W., Grinberg, A., 2013. Fecal pH and Microbial Populations in Thoroughbred Horses during Transition from Pasture to Concentrate Feeding. J. Equine Vet. Sci. 33, 215-222.
11.
P. Grimm, J.P. Pais de Barros and V. Julliand Impact of diet on bacterial lipopolysaccharides in equine faeces, EWEN 2016.
Report on the European Workshop on Equine Nutrition (EWEN): Part 2
Founder of MB Equine Services, Mariette van den Berg, travelled in June to Europe to attend two very special equine science conferences – the European Workshop on Equine Nutrition (EWEN) and the International Society for Equitation Science (ISES) Conference.
 At both of these conferences, Mariette presented results from her PhD project that focuses on behavioural mechanisms of diet selection by horses. In this series, she features a selection of interesting topics and findings from her own project, and other researchers who attended the 8th bi-annual EWEN, which was held in Dijon, France.
At both of these conferences, Mariette presented results from her PhD project that focuses on behavioural mechanisms of diet selection by horses. In this series, she features a selection of interesting topics and findings from her own project, and other researchers who attended the 8th bi-annual EWEN, which was held in Dijon, France.
EWEN is recognised as Europe’s leading scientific event in equine nutrition, and brings European and international scientists together to share scientific information, disseminate technical expertise amongst the wider horse industry and foster innovation.
In this, Part Two of her report, she presents a summary of a study that investigated whether plant secondary compounds and additional protein in the diet could help control strongyle infections.
2. Researchers investigate alternative worm control
Due to the alarming spread of worms that have developed a resistance to current anthelmitic drugs and the lack of new drugs being developed, research teams around the world have been looking for alternative treatments.
A French research group examined the use of secondary plant compounds (condensed tannins) and extra protein in the diet of horses as an alternative to control strongyle (redworms) infections. In earlier articles on pasture diversity, and fodder trees and shrubs for horses, I have written about the benefits of plant secondary compounds, and it is great to see more research is being done on this subject.
Plant secondary compounds are complex chemicals made by plants that are not normally essential to the life of the plant. They are thought to be produced for the plant’s protection, for example as pesticides and anti-grazing agents, but they also include pigments, hormones and chemical agents that can attack other plants (alleleopathy).
The French presentation was titled: Sainfoin (Onobrychis viciifolia) or extra proteins in the diets as an alternative to control horse strongyle infections? (G. Fleurance et al.).
Anthelmintic resistance of equine strongyle nematodes to regular wormers has become a major problem worldwide and has created an urgent need for more alternative strongyle control.
In ruminants, it has been suggested that extra protein (improved feed complementation) could help animals compensate for the parasite burden, especially if diets are protein-limiting, and the use of bioactive forages, like sainfoin, which contains plant secondary compounds (condensed tannins), have been proven to have anthelmintic effects in several studies in small ruminants (Houdijk et al. 2000, Brunet et al. 2007).
In equines, only two in vitro (in the lab, rather than in horses) studies have been conducted that investigated the influence of different plant extracts and demonstrated a significant effect against strongyle for a number of them (Payne et al. 2013, Peachey at al. 2015). Therefore, the aim of this study was 1) to evaluate the efficiency of a short-term consumption of either extra protein or sainfoin by horses to reduce faecal egg excretion in naturally-infected horses and, 2) to investigate the influence of secondary compound of sainfoin on strongyle egg hatching and larval development in vivo (in horses).
The experiment was conducted at an experimental farm of the French Horse and Riding Institute in Chamberet, France, over a period of 17 days in 2013. The researchers used 30 horses that were divided into three groups of 10 individuals.

Group one received a tannin-rich diet (SD), with 70% dry matter (DM) sainfoin pellets (which would contain 3.6% DM condensed tannins). Group two was offered a protein-rich diet (PD), with 52% DM Italian rye-grass pellets and 10% DM grinded linseed expeller. Group three received a control diet (CD) with 45% DM barley and 25%DM cereal-based pellets. All groups were given 30% wheat straw.
The diets were all similar in energy intake, covering an average of 94% of energy requirements. SD and PD diets provided extra protein – about 227% of protein requirements vs. 93% for CD diet. The researchers compared PD and CD to test for benefits of extra protein, while SD and PD were compared to examine the effect of the sainfoin secondary compound.
Faecal egg counts (FEC) were individually measured on four dates (horse grouping, adaptation period, and start and end of experiment). They also investigated larvae development (L3) by incubating (pooled) faecal samples and they performed two in vitro experiments; a larvae development assay with rehydrated sainfoin at different rates (0-29%), which was added to faeces of infected horses (6 replicates) and an egg-hatching assay, which involved adding sainfoin solutions at different concentrations to strongyle eggs solutions (5 replicates).
While this study reported that overall FEC decreased over time, there was no significant effect of diet or diet x sampling time interaction. In addition, they did not report the baseline FEC levels per group, so it is difficult to assess if there were any differences within groups.
However, at group level, the authors did observe a lower rate of strongyle larval development in the SD group at the end (SD: 8%, PD 30.5% and CD: 22.6%), but they were not able to perform any further analysis to determine if this difference was significant, as they had no replicates.
Nevertheless, they were able to confirm that adding 29% sainfoin pellets to the faeces (manure) significantly reduced strongyle egg development into larvae (L3) – by 82% – and a solution with sainfoin concentrations of 7.5 mg/ml or higher significantly reduced egg hatching – by 36%.
These findings suggest adverse effects of sainfoin on larvae development, but more in vivo work needs to be conducted to replicate the results for the larvae development, as well as for the effect on FEC when the sainfoin is added to the diet.
It is also not clear if the extra protein had an effect in this study, so they suggest more work needs to be done with more protein-limiting diets. Nevertheless, this study suggests that short-term use of sainfoin in horse diets could be a promising strategy to reduce risk of infection by strongyles at pasture.
If you are interested in more information about this topic, check out the Australian paper by Payne et al. 2013 ‘Australian plants show anthelmintic activity toward equine cyathostomins in vitro’, which you can read here.
References:
- Brunet, S., Aufrere, J., El Babili, F., Fouraste, I., & Hoste, H. (2007). The kinetics of exsheathment of infective nematode larvae is disturbed in the presence of a tannin-rich plant extract (sainfoin) both in vitro and in vivo. Parasitology, 134(09), 1253-1262.
- Houdijk, J. G. M., Kyriazakis, I., Jackson, F., Huntley, J. F., & Coop, R. L. (2000). Can an increased intake of metabolizable protein affect the periparturient relaxation in immunity against Teladorsagia circumcincta in sheep?. Veterinary Parasitology, 91(1), 43-62.
- Payne, S. E., Kotze, A. C., Durmic, Z., & Vercoe, P. E. (2013). Australian plants show anthelmintic activity toward equine cyathostomins in vitro. Veterinary parasitology, 196(1), 153-160.
- Peachey, L. E., Pinchbeck, G. L., Matthews, J. B., Burden, F. A., Mulugeta, G., Scantlebury, C. E., & Hodgkinson, J. E. (2015). An evidence-based approach to the evaluation of ethnoveterinary medicines against strongyle nematodes of equids. Veterinary parasitology, 210(1), 40-52.
European Workshop for Equine Nutrition 2016 (Part 1)
 Founder of MB Equine Services, Mariette van den Berg, travelled in June to Europe to attend two very special equine science conferences – the European Workshop on Equine Nutrition (EWEN) and the International Society for Equitation Science (ISES) Conference.
Founder of MB Equine Services, Mariette van den Berg, travelled in June to Europe to attend two very special equine science conferences – the European Workshop on Equine Nutrition (EWEN) and the International Society for Equitation Science (ISES) Conference.
At both these conferences, Mariette presented results from her PhD project that focuses on behavioural mechanisms of diet selection by horses. In this new series, she will be featuring a selection of interesting topics and findings from her own project, and other researchers who attended the 8th bi-annual EWEN, which was held in Dijon, France.
The bi-annual European Workshop on Equine Nutrition was first held in 2002 in Dijon and, after 2004, has been touring through Europe, visiting Italy in 2006, Finland in 2008, the United Kingdom in 2010, Portugal in 2012 and Germany in 2014, before returning again to France this year.
EWEN is recognised as Europe’s leading scientific event in equine nutrition, and brings European and international scientists together to share scientific information, disseminate technical expertise amongst the wider horse industry and foster innovation. Each conference has a theme and this year EWEN 2016 was entitled ‘Taste, Nutrition and Health of the Horse’.
 This focus was chosen because of the location, which is home to the Centre for Taste, Food and Nutrition Sciences (CSGA). Of course, Dijon is also very conveniently located in the Burgundy region, which is famous for its wine and great cheeses!
This focus was chosen because of the location, which is home to the Centre for Taste, Food and Nutrition Sciences (CSGA). Of course, Dijon is also very conveniently located in the Burgundy region, which is famous for its wine and great cheeses!
‘Taste’ is a conference topic which has not been studied extensively in horses and is very applicable to my PhD project, which investigated factors that influence foraging behaviour and diet selection in horses, with a main focus on orosensory (smell, taste, texture) and post-ingestive (nutrients) feedback mechanisms.
This month, I present a summary of two presentations, the first is one of my presentations that described some of the findings from our peer reviewed paper entitled ‘Influence of odour, taste and nutrients on feeding behaviour and food preferences in horses’. This full study has been recently published in the Applied Animal Behaviour Science Journal, which you can access here. The second is a fascinating study aimed at finding alternative wormers to fight the alarming resistance parasites are developing to anthelmitic drugs and tested plant secondary compounds.
1. Effect of flavours and odours on diet intake in horses
In one of our earlier studies that examined the acceptance of nutritious novel forages, we had observed horses almost invariably consume only small quantities of food when it is presented for the first time (van den Berg et al., 2016a). The cautious sampling of new food types is referred to as neophobia (‘fear of new’) and has been suggested as an innate herbivore survival mechanism for avoiding the over-consumption of toxic plants in the wild (Provenza and Balph, 1988).
 While feed neophobia is usually described in horses as “fussy eaters”, similar behaviour occurs in many animal species, including humans! Commercial horse feed manufacturers commonly use flavours to overcome this feed neophobia, but there are limited studies that have investigated how well horses accept these flavours.
While feed neophobia is usually described in horses as “fussy eaters”, similar behaviour occurs in many animal species, including humans! Commercial horse feed manufacturers commonly use flavours to overcome this feed neophobia, but there are limited studies that have investigated how well horses accept these flavours.
Flavours have been added to encourage the intake of water, unpalatable anthelmintic drugs (wormers), as well as concentrates and supplements with different effects. For example, a study by Burton et al. (1983) used apple, lucerne, caramel and anise-molasses to mask the drugs levamisol and piperazine in a concentrate diet, and showed only apple, lucerne and caramel were partially effective, not anise-molasses.
Goodwin et al. (2005a) examined the effect of 15 food flavours on concentrate selection by stabled horses and showed the two most preferred flavours (fenugreek and banana) could be used to encourage the intake of an unpalatable mineral pellet. However, the characteristics of what flavours are most useful have not been well defined.
For example, flavours can be classified as non-nutritive, providing only an aromatic and/or non-caloric taste (artificial or natural sweetener), or nutritive, which can include an aromatic and/or taste that contains calories.
In another study, Goodwin et al. (2005b) reported in multiple choice trials and in the short term, horses can respond to sensory variety in concentrate diets that differ in flavours (odour and caloric taste) and/or formulations (nutrients), and they select from preferred and less preferred food choices.
Nevertheless, it was still unclear how horses respond to non-nutritive flavour changes over a longer period when they are presented with a single diet. This is why, as part of a larger study and over a period of four weeks, we examined the responses to flavour (odour) changes on diet intake. Our main objective was to assess the diet/odour acceptance and variability in intake after each diet/odour introduction was made.
In our study, we offered four fibre pellet diets to 16 adult horses. These diets were similar in digestible energy, but differed in crude protein (CP) levels (Low CP; 14% and High CP; 27%), with one of these levels including a non-caloric natural sweetener + taste.
 The four diets (LP, LP+, HP and HP+) were presented in the order from low to high palatability over four weeks (five days on each diet) and were linked to four non-caloric odours (spearmint, banana, cinnamon and coconut).
The four diets (LP, LP+, HP and HP+) were presented in the order from low to high palatability over four weeks (five days on each diet) and were linked to four non-caloric odours (spearmint, banana, cinnamon and coconut).
These four odours were chosen from different flavour classes (i.e. nut flavour, fruit flavour, spice flavour and herb flavour) to avoid similar characteristics and confuse horses. The aim of the adaptation phase was to allow horses to learn about the flavour-to-post-ingestive associations of each diet – this was important for later preference testing and diet ranking.
To make sure all diets and odour combinations were tested, the horses were divided into four groups, which allowed for randomisation of the odours to the four diets (Latin Square 4 x 4). This meant each group received its own unique odour-diet combinations throughout the adaptation phase and during further preference testing.
The odours were sprayed onto the feed before being presented to the horses. Horses were offered 400g per diet/day. The intake was recorded daily and calculated to proportions consumed out of the total food offered.
Our results showed with each introduction of a new odour/diet, an initially large variation in intake was recorded, with some horses showing a neophobic response, while others exhibited no apparent recognition of the odour being new.
Individual variability reduced within 3-4 days for all four odours, with horses consuming between 80-100% of the diet.
 This adaptation of 3-4 days to new diet / odour was also observed in our previous study (van den Berg et al., 2016b); suggesting odour cues can have a strong initial influence on feed intake by horses and highlights the important role of smell in diet selection.
This adaptation of 3-4 days to new diet / odour was also observed in our previous study (van den Berg et al., 2016b); suggesting odour cues can have a strong initial influence on feed intake by horses and highlights the important role of smell in diet selection.
Even so, we conclude that non-nutritive odours can be safely introduced to equine diets without upsetting intake patterns for prolonged periods of time. In addition, once horses are familiar with specific odours (that are linked to well-liked diets), you can use this odour as a cue to ease the transition from familiar food to more novel or less palatable foods.
References
- Burton J.H., Price D.J. and Aspinal J. 1983. The effect of feed flavour and feed consumption in horses. In Eight Equine Nutrition and Physiology Symposium Lexington, KY, USA.
- Goodwin D., Davidson H.P.B. and Harris P. 2005a. Selection and acceptance of flavours in concentrate diets for stabled horses. Applied Animal Behaviour Science 95, 223-232.
- Goodwin D., Davidson H.P.B. and Harris P. 2005b. Sensory varieties in concentrate diets for stabled horses: effects on behaviour and selection. Applied Animal Behaviour Science 90, 337-349.
- Provenza F.D. and Balph D.F. 1988. Development of dietary choice in livestock on rangelands and it’s implications for management. Journal of Animal Science 66, 2356-2368.
- van den Berg M., Lee C., Brown W.Y. and Hinch G.N. 2016a. Does energy intake influence diet selection of novel forages by horses? Livestock Science 186, 6-15.
- van den Berg M., Lee C., Brown W.Y., Giagos V. and Hinch G.N. 2016b. Acceptance of novel food by horses: the influence of food cues and nutrient composition. Applied Animal Behaviour Science, 183, 59-67.
Equine Podiotherapy Conference/ Bowker Lectures 2015
 The Australian College of Equine Podiotherapy conducts a biennial conference that aims to present current, progressive, objective and scientifically justified information to equine industry professionals (vets, vet chiros, body therapists, equine podiatrists as well as ‘progressive’ horse owners). Our aim is to disseminate information covering all aspects of horse management that is slanted towards keeping horses sound and in optimum health for the long term, not just next weekend’s blue ribbon.
The Australian College of Equine Podiotherapy conducts a biennial conference that aims to present current, progressive, objective and scientifically justified information to equine industry professionals (vets, vet chiros, body therapists, equine podiatrists as well as ‘progressive’ horse owners). Our aim is to disseminate information covering all aspects of horse management that is slanted towards keeping horses sound and in optimum health for the long term, not just next weekend’s blue ribbon.
The conference is named in honour of our long term mentor Professor Robert Bowker from Michigan State University whose research has fostered a generational change in equine hoof management.
Our next conference is scheduled for 21-23 February 2015 in North East Victoria (at Pinnacle Valley Resort near Mansfield).
In addition to Prof. Bowker who will be presenting his latest research findings relevant to equine hoof function, we have secured Dr Kerry Ridgeway to present his latest thoughts relative to equine body therapy, Dr Ann Nyland to speak on equine intestinal health, Dr Penelope Thomson to speak about the current pain relief options for lame horses, Sharon May Davis to present a couple of new peer reviewed research papers relevant to functional anatomy, Mariette van den Berg (MB Equine Services) to speak about integrated pasture management and equine foraging behaviour and a half dozen hoof specialists who will discuss current ‘in the field’ research they are conducting.
Something new for us at this conference (with the three days allowing us to have an official dinner) will be an after dinner speaker. We are excited to announce that Dr Andrew McLean from the Australian Equine Behaviour Centre, will be our guest speaker. He will talk bout “Ethology and Learning Theory in Horse Training”.
It doesn’t matter what corner of the equine therapy world we work in, be it hooves or body or even veterinary medicine, the more we look at the whole horse, the better are the long term results.
The best trimmers are those who learn about bodies. The best body therapists are those who learn about the hooves.
There are also the amazing networking opportunities generated by such a gathering of like minds. You never know just who you may meet at a conference like this.
 The venue at Pinnacle Valley is simply breathtaking. Right at the very foot of the iconic Victorian High Country. If you are from interstate you may wish to include a high country horse ride in your itinerary. February is the best time to experience the high country.
The venue at Pinnacle Valley is simply breathtaking. Right at the very foot of the iconic Victorian High Country. If you are from interstate you may wish to include a high country horse ride in your itinerary. February is the best time to experience the high country.
If you are intending to participate, don’t leave it to the last minute, numbers are limited. Please call or email in for your name to be put on the list. As soon as registration documents are completed they will be emailed out to you.
The cost for the three days is a very affordable $475. This includes morning tea afternoon tea and lunch. The dinner will be extra. As further details are cemented, we will update you on the Barefootblacksmith Facebook site and our web site http://www.barehoofcare.com/index.html
Info:
Date : Saturday 21st, Sunday 22nd and Monday 23rd February 2015
Venue : Pinnacle Valley Resort, Merrijig, Victoria
www.pvr.com.au
Time : Saturday – Registrations – 8.30 am
Lectures – 9.00 am – 6.00 pm Saturday and Sunday
Dinner & Guest Speaker – 6.00 pm Sunday
Lectures – 9.00 am – 3.00 pm Monday
Cost : $475 – Conference proceedings book, morning
tea, afternoon tea and lunch included.
Dinner$45. Total inc. Dinner $520
Registrations open 2 1 s t October 2014. Positions filled in order of payments received.
Complete attached registration form or download at www.barehoofcare.com and fax to 03 5773 4307.
You can also register in person at Equitana 2014
Download here:
Equine Health Workshop: South East QLD (Canungra) – 1st of June 2014
 Equine Health Workshop: South East QLD
Equine Health Workshop: South East QLD
When: 1st June 2014
Where: Canungra – School of Arts Hall
Time: 8:30-12:00
Cost: FREE
Topics:
– Equine Nutrition
– Gastrointestinal Tract
– Digestive & Metabolic Disorders
– Nutrient Resources & Nutrient Requirements
– Dietary Management
– Parasite Control
– Biosecurity QLD – Hendra Update
Booking and more information: http://www.seqcatchments.com.au/announcements/horse-nutrition-canungra



Follow Us!